
In 1896, Henri Becquerel discovered a new mysterious radiation. Unlike X rays that disappearead when the X-ray tube was switched off, this new radiation observed from Uranium was being emitted continuously. Further studies by Pierre and Marie Curie revealed that other elements had the same property. In particular they found these properties in polonium and radium in 1898.
Pierre and Marie Curie quickly found a use for this property and radium was being used in hospitals as early as 1901. It was recognised that the radium radiation has beneficial effects on many skin diseases including cancer.
Pierre and Marie Curie in their laboratory, Paris 1898.
The new radiation was of nuclear origin. Some nuclei are spontaneously emitting α particles (helium nuclei), β particles - these can be electrons (β-) or positrons (β+) - and/or γ (gamma) rays (i.e. high-energy photons). Such an atom with an unstable nucleus, characterized by excess energy is called a radionuclide or radioactive nuclide, and also referred to as a radioisotope or radioactive isotope.
Natural radioisotopes are for example 238U (uranium), 40K (potassium), 232Th (thorium) and their daughter nuclei 226Ra (radium), 222Rn (radon), 218Po (polonium). Some radioisotopes are produced continuously by the action of cosmic rays on the upper layers of our atmosphere, like, for example, 14C (carbon), 3H (tritium) or 7Be (beryllium).
Irene and Frederic Joliot-Curie were the first to produce artificial radioisotopes in 1933, by using a natural α-source to bombard aluminium-27 and produce the radioelement phosphorus-30 that β-decays to silicon-30. This was the result of the nuclei of the aluminum atoms acquiring additional protons from the helium nuclei (α particles).
Since then, many new radioisotopes have been produced, opening up a number of new applications and leading to a better understanding of nuclear matter and nuclear forces. By 1936, some 200 radio-nuclides had been produced. Today about 250 stable nuclides are known. They’re produced using beams of particles, ranging from protons to 238U, accelerated in cyclotrons or linear accelerators.
The first accelerator (cyclotron) was built at Berkeley, in the USA in 1932 by Ernest O. Lawrence. He and his brother John Lawrence, a doctor, soon recognised very important medical applications and produced radioactive isotopes for biological and medical research, as well as for the treatment of cancers. The first European cyclotron was built by Frederic Joliot (1939) at College de France, Paris.
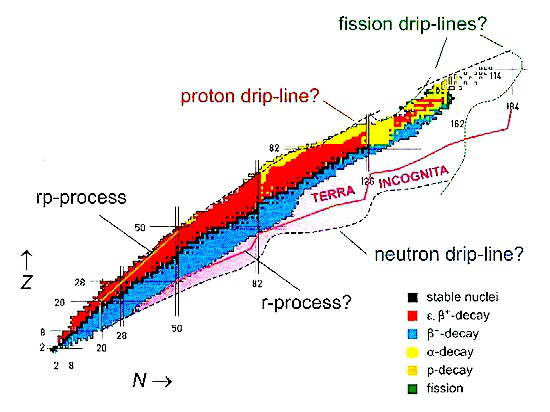
Physicists have produced many new isotopes that are further and further away from the natural nuclear stability valley. At present, there are many facilities employing radioactive ion beams. The demand for medical radioisotopes is a worldwide phenomenon, and in the developed countries it has been growing exponentially since 1995.
The 1943 Nobel prize in Chemistry was awarded to G. de Hevesy for being the first to use natural (and later artificial) radioactive elements as tracers to study stable elements in biological systems. A very small amount of a radioactive element can easily be followed in a human body. This can also be achieved via chemical tracers, but far larger amounts of chemicals would be needed, making the procedure far riskier for the patient.
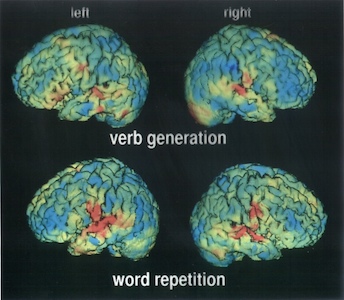
In a typical nuclear-medicine investigation a tracer is administrated to the patient and the γ rays emitted by the tracer nuclide are recorded with an array of radiation detectors. This is called emission tomography. The tracer is usually chosen such that it is deposited selectively in a particular organ, and then the detected γ rays provide a detailed image of the region of interest. To the trained eye, the images can reveal structural or metabolic abnormalities leading to better diagnoses. In nuclear medicine a large variety of isotopes are employed, each specifically targeted for individual organs of the human body.
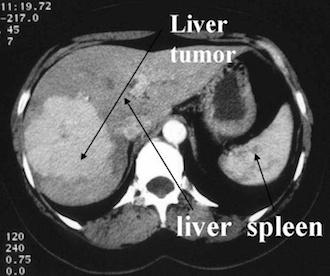
Image above: PET application in neurophysiology (verbal working memory in the brain), San Raffaele Hospital, Milan.
Tumours can be located with techniques like Single Photon Emission Computed Tomography (SPECT) or Positron Emission Tomography (PET) (in which positron (β+) emitting nuclei are used to produce - through annihilation process - energetic γ rays). SPECT and PET techniques are standard in tumour diagnosis as well as in functional studies of the normal healthy brain.
Image from a computerised tomography using X-rays from a iodine tracer (Hospital Erasme, Bruxelles).
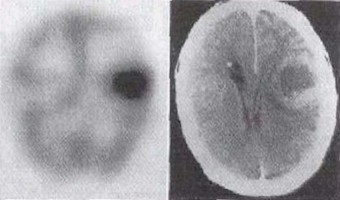
Among the radioactive elements, iodine (I) plays an exceptional role, as it attaches itself to the thyroid tissue with great affinity and selectivity. In low doses it can be used for diagnosis purposes, or, alternately radiation emitted from radioactive iodine exclusively attached to the thyroid tissues can treat thyroid cancer.
The physiologically most interesting chemical elements like C (carbon), N (nitrogen) and O (oxygen) have β+-emitting short lived isotopes used in PET techniques. The commonly used beta-emitting nuclides are 11C, 13N, 15O and 18F (fluorine).
Image right: Scintigraphy of thyroid activity using technetium.
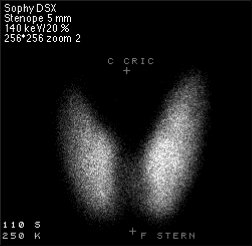
SPECT stands for Single Photon Emission Computed Tomography. In this procedure, organs are imaged by measuring the distribution of an injected radio-tracer by means of a gamma 'camera' linked to a computer. SPECT imaging involves the rotation of a gamma detector array around the patient to acquire measurements from multiple angles. With this technique, the position and the concentration of radionuclide distribution is sought. SPECT involves the use of radioisotopes like 99mTc, or 123I where a single γ (of 140 keV) is emitted.
There are many applications of SPECT both in and as a therapy aid. SPECT scans using a radioisotope not absorbed by tissues but instead travelling in the bloodstream can be used to see how blood flows to certain tissues and organs. This is particularly useful for scans of the brain and heart.
In the Positron Emission Tomography (PET) technique, commonly used isotopes are 15O, 13N, 11C and 18F. These have short half-lives of about 2, 10, 20.4 and 110 min, respectively, so the radioisotopes have to be produced close to where they’re used. For this reason, many hospitals have a small cyclotron nearby or even within the hospital itself, specially constructed to produce these isotopes. The isotope is then attached to a natural chemical such as glucose, water or ammonia which is then put into the human body via an injection or possibly by breathing it in as a gas. The radioisotope travels to the part of the body that uses the chemical it was attached to, which is why many different types of chemicals are used.
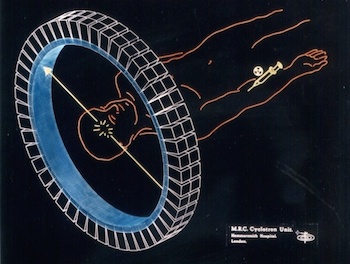
Elementary particles have corresponding anti-particles. A particle’s anti-particle is effectively the same particle but with the opposite electrical charge. When a particle and its anti-particle collide, they annihilate with the emission of pure energy.
The isotopes typically used in a PET scan are β+ emitters. When the isotope decays inside the patient's body, any emitted positron (β+) travels only a short distance in the tissue (typically less than 1 mm, but this depends on the isotope) before it annihilates with an electron (β-). The energy released at the annihilation is in the form a pair of gamma (γ) photons emitted at 180° with respect to each other, and an energy of 511 keV each. The detection of the two γ rays in coincidence by two detectors allows one to localise the decay event on the line connecting the two detectors.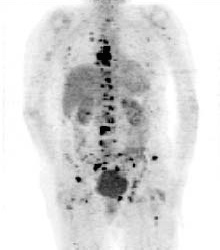
PET scans are commonly used to diagnose cancers, heart conditions and some brain disorders like epilepsy or Alzheimer’s disease. PET is also employed in assessing the response to cancer therapy. In addition, PET scans are used in research, for example studies of the effects of drug abuse or ageing.
The risks associated with a PET scan are very small but as with most types of scans, it’s not always suitable for pregnant or nursing women. Due to the presence of a radioactive substance in their body, hospitals advise patients to not to have any close contact with pregnant women or young children for at least a few hours after a PET scan.
PET scan image showing bone cancer of the skeleton (Hospital Erasme, Bruxelles).SPECT imaging is inferior to PET in terms of attainable resolution and sensitivity. For PET the best spatial resolution attaineble presently is of about 6 mm, that is 2 to 3 times better than with SPECT. Because of their rapid decay, essentially all PET isotopes must be produced on the premises (with the exception of 18F of 110 min half-life). In contrast, SPECT isotopes like 123I have a sufficiently long half-life (13.2 hrs) to allow centralised production at distant sites with delivery then via express mail.
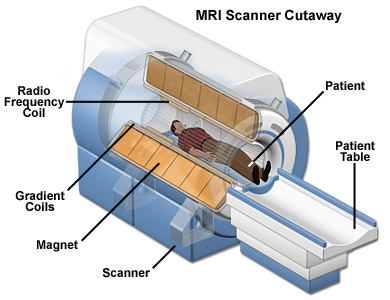
The main benefit of MRI is that it’s doesn’t use any radioactive substance. MRIs show the distribution of water (H2O) in the body and the organs where it is stored. The MRI is basically a large tube with powerful magnets inside. This is because the protons in the H nuclei behave like tiny magnets. When an external magnetic field is applied around the patient (which is not harmful) all those tiny magnets in the water are pulled in one direction. When the proton snaps back into position it releases radio waves which the MRI scanner can then detect and measure to make a 2D image of the body. MRIs are commonly used for brain or heart diagnoses but can be used to scan almost any part of the body.
MRI technology is further being developed to address a wider range of clinical diagnosis problems. For example, polarised Xe-129 or He-3 can be used to image the airways in the lungs with high spatial and temporal resolution.
The 2003 Nobel Prize for medicine was awarded to to Paul Lauterbur and Sir Peter Mansfield for their work and contribution to Magnetic Resonance Imaging.
Most modern and academic hospitals nowadays employ MRI and PET, along with other imaging techniques. In many Nuclear Physics institutes there are groups whose activities and research are devoted to specific medical applications as well as to patient treatment, for example at GSI (Germany), PSI (Switzerland), GANIL (France), HMI (Germany) or LARN (Belgium).Particle therapy involves aiming ionised particles at the targeted tumour. The ionization indiscriminately damages cell DNA. However, cancer cells are less able to repair themselves and are more affected by this damage.
The particles used include protons, neutrons or positively charged ions. Each type of particle has a different energy and can therefore penetrate the human body by different amounts. This is why different types of particles are used for different procedures. The most common type of particle therapy is proton therapy.
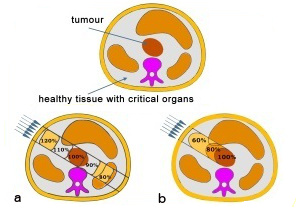
A fundamental problem of radiation therapy is the need to maximize the dose delivered within to the tumour while minimising harm to the healthy tissue around it. In electromagnetic radiations (X or γ rays), a large fraction of the dose affects the healthy tissue along the fascicle in front of and behind the tumour.
An improvement in radiation therapy is now achieved using high energy beams of particles (p, heavy ions) because for ion beams the doses increase with the penetration depth up to a maximum at the end of the particle range. Ion beam therapy allows a larger and more effective dose to be deposited in the tumour than any other type of external therapy.The image above right shows a comparison of two procedures. Photon therapy (a) deposits a high dose of radiation in the healthy tissue in front of the tumour. In the case of a particle beam (b), most of the dose is confined to the tumour.
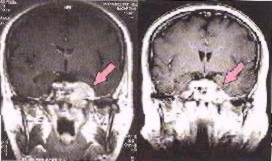
Image left shows a brain cancer scan before, and six weeks after radiotherapy with photons and Carbon ions.
To date, more than 50 000 patients worldwide have been treated with proton beams. The treatment of eye tumours by proton beams yields by far the best clinical result. Proton therapy is also ideal when the tumour is close to a critical organ as it can be aimed very accurately. The better conformity of the irradiated volume with the target volume, the better the results. This also holds true for heavy ions beams, where a superior conformity compared to protons can be achieved. Heavy ion therapy was pioneered in 1974 at Lawrence Berkeley Laboratory. The first treatments made use of Ar (argon) ions and then later, Si (silicon) and Ne (neon) ions. The irradiated tumours were close to critical organs in the brain, head or neck. The results were excellent and motivated the construction of dedicated Heavy Ion Medical Accelerators.
Brachytherapy is a form of radiation therapy where radioactive material is placed inside the body, close to the tumour. The advantages of this procedure are that the radiation does not have to travel from the outside of the body through healthy tissue to reach the tumour and instead only affects a localised area. This means that relatively high doses can be given with minimal risk. Also, the patient is mobile during the length of the treatment. The overall treatment is usually completed in less time than other procedures and so there’s less time for cancer cells to multiply in between treatment sessions.
Brachytherapy is a standard technique for some gynaecologic cancers such as cervical or prostate cancer and certain stages of head and neck carcinoma. For prostate diseases, various approaches have been used and these are mainly dictated by the isotope selected. 125I (with a half life of 60 days) and 103Pd (with half life of 17 days) are used for permanent implants. These isotopes can be produced by neutron capture in reactors. 103Pd can be produced more efficiently in cyclotrons (with proton beams of 14 MeV). More than 10 years of clinical data from the use of 103Pd in prostate brachytherapy exists in the USA. This clinical success, together with a lack of side effects, has made it a very popular choice for therapy.
The Sun emits visible light, infrared radiation that we feel as heat, and ultraviolet radiation that, unfortunately, can be dangerous. The ultraviolet radiation can cause skin melanoma (skin cancer). Skin melanoma is one of the most common causes of cancer and in 2008 for example has killed about 46,000 people worldwide.
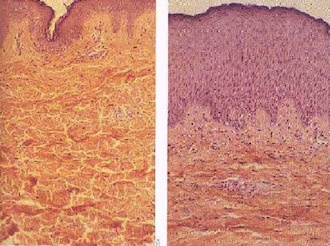
The rate of malignant melanoma diagnoses has quadrupled in the last 30 years. It is most commonly found in people with a family history of skin cancer, fair-haired and fair-skinned people and those older than 40. However, anyone can develop cancer regardless of race, age or genetics. Most skin damage occurs before the age of 20 but often doesn’t show up until the age of 40 so even if you can’t see yourself burning, you’re still damaging your skin. This is why it’s so important to wear sun cream and stay out of the Sun when it’s strongest (between 11am and 3pm).
