
As early as February 1896, X-rays were used in medicine to observe broken bones: the first such observation, of a fracture in a patient's arm, was carried out by two physicians at Dartmouth College in the USA. Quite soon, other applications of X-rays were found, including luggage scanning by custom officers.


X-rays attracted the attention of many physicians who then employed these rays to diagnose fractures and foreign bodies inside tissues. At the end of the 19th century, medicine was paying particular attention to the pathology of the individual organs. Continuous improvements allowed physicians to take X-ray photographs of all internal organs by means of “contrast media” - opaque substances which showed up in X-rays. X-rays were also used in therapy, for example in the treatment of skin diseases. However, it was soon realised that radiation of higher energy was required for this type of therapy.
X-rays really came into their own during the First World War. Marie Curie together with her daughter Irene set up a network of medical radiological centres to help improve the diagnosis of fractures and lung diseases among soldiers. In addition, many specialized vehicles with X-rays apparatuses (called “Les Petites Curies”) were covering the battlefields. Marie Curie authored a book about this: “Radiology in War” (1921).
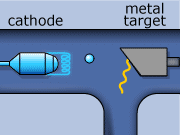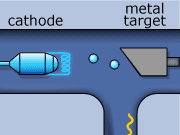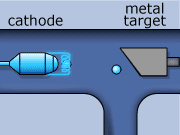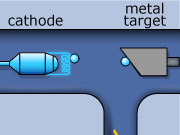
These rays are partly a result of the fluorescence excited in the atoms of the metal, and partly of the so-called Bremsstrahlung radiation effect. The latter appears as the result of rapid changes of direction of the electros
in the vicinity of the atomic nuclei of the metal.
Another way in which X-rays can be produced is when the initial high energy electron knocks out one of the electrons from the inner electron shell. Then one of the higher energy electrons will drop down on the inner shell to fill the gap and will emit its excess energy in the form of an X-ray.
It is worth comparing these contributions:
| Source | Equivalent dose |
| Chest X-ray | 100 μSv |
| Living in a stone, brick or concrete building for one year | 70 μSv |
| Flight from London to New York | 40 μSv |
| Average daily natural background dose | 10 μSv |
| Dental X-ray | 5 μSv |
| Eating one banana | 0.1 μSv |
As you see, even eating a banana exposes one to a radiation dose. The main source of this is the potassium, which in nature contains 0.0117% of the unstable isotope 40K.
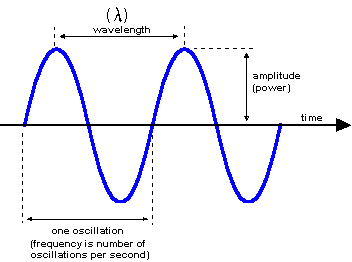
Every form of electromagnetic radiation is characterised by a wavelength (λ) or equivalently by an energy (E = hc/2λπ, where h is the Planck constant (h = 6.626×10-27 erg/s), c is the speed of light in vacuum (c = 2.9979·1010 cm/s).
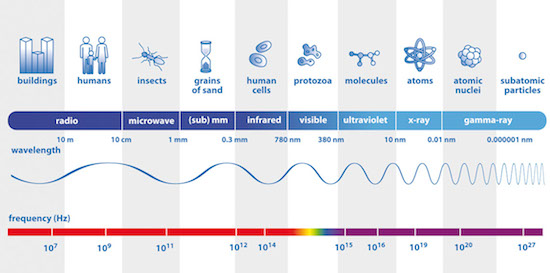
The electromagnetic spectrum, from radio waves to γ rays (Image credits: ESA).
