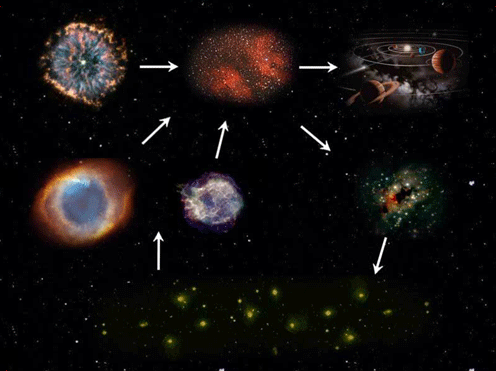
To put it plainly, without stars, we simply would not exist. In fact, there probably wouldn’t be any life at all. But why are stars so essential?
The most obvious answer is of course, energy. Stars produce the energy necessary for life by nuclear fusion. If stars would have never formed in the Universe, there would be eternal darkness and unimaginably cold temperatures, close to -273˚c. Our Sun releases billions of watts of power and helps to heat up the planet Earth, creating just the right temperature for liquid water and subsequently, for life.But perhaps even more importantly, stars create many of the essential elements necessary for life. Most of the important molecules in our bodies are made from carbon. We also need oxygen in water, calcium in our bones, iron in our blood and much more. All of these elements are created inside the stars. When stars die these elements are released into space to be reformed as almost everything we know on Earth. We are all literally made of stars!
For most of their life, stars burn hydrogen and fuse hydrogen nuclei into helium nuclei. There are two different ways to do this. The first is called the pp-chain, the second is called the CNO-cycle.
Hydrogen-burning stars are called Main Sequence stars, of which we learned in the previous chapter. Our Sun is a Main Sequence star, with most of the energy created via the pp-chain, about 98.5%, and the other 1.5% created via the CNO-cycle. This is typical for a star of Sol's mass. The pp-chain is the most dominant nuclear process for main-sequence stars with masses up to about 1 and a half times the mass of our Sun. Any mass higher than that would allow the CNO-cycle to dominate. By now, the Sun has burned approximately half of the hydrogen in its centre and it is about 4.5 billion years old.
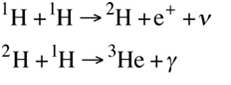 The pp-chain always starts with two hydrogen nuclei and produces helium-4. Hydrogen nuclei (which would make a hydrogen atom if they were accompanied by an electron) are effectively just single protons, p. So, the two protons fuse together to form deuterium, releasing a positron and a neutrino as one proton turns into a neutron. The resulting deuterium then fuses with another proton to create an isotope of helium, helium-3, releasing also a photon.
The pp-chain always starts with two hydrogen nuclei and produces helium-4. Hydrogen nuclei (which would make a hydrogen atom if they were accompanied by an electron) are effectively just single protons, p. So, the two protons fuse together to form deuterium, releasing a positron and a neutrino as one proton turns into a neutron. The resulting deuterium then fuses with another proton to create an isotope of helium, helium-3, releasing also a photon.
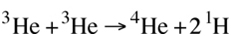
This is occurring throughout the star so there are a relatively high number of helium-3 molecules available. From here, there are actually three different ways to produce helium-4. The ppI-chain produces 86% of the Sun’s energy. In this process, two helium-3 nuclei fuse to produce helium-4 and the left over protons are released to be eventually part of another pp-chain reaction.
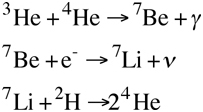 The ppII-chain produces about 14% of the Sun’s energy. In this process, one of the helium-3 molecules combines with an already existing helium-4 molecule to produce beryllium-7 and a photon. However, beryllium-7 is unstable and decays via electron capture, that is, it combines with an electron to form lithium-7, and releasese a neutrino in the process. Finally the lithium-7 combines with a deuterium molecule to create two helium-4 molecules.
The ppII-chain produces about 14% of the Sun’s energy. In this process, one of the helium-3 molecules combines with an already existing helium-4 molecule to produce beryllium-7 and a photon. However, beryllium-7 is unstable and decays via electron capture, that is, it combines with an electron to form lithium-7, and releasese a neutrino in the process. Finally the lithium-7 combines with a deuterium molecule to create two helium-4 molecules.
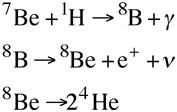
There's also the ppIII-chain, only responsible for 0.02% of the Sun’s energy. Here, the unstable beryllium-7 combines with another proton to form boron-8 and a photon. Boron-8 is highly unstable and quickly decays to form another unstable isotope of beryllium, beryllium-8, releasing a positron and a neutrino as byproducts. Beryllium-8 decays back down to two helium-4s.
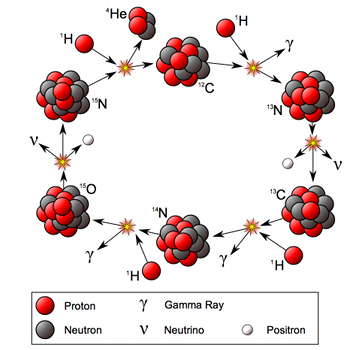
The nuclear processes taking place in the Sun, the pp-chain and the CNO-cycle, create a lot of products that we’ve yet to talk about. Obviously, the energy produced is mostly photons, which we see as sunlight. However, the nuclear processes also produce a lot of neutrinos.
Physicists find neutrinos very intriguing: they are very unusual particles. Neutrinos carry a lot of energy but don’t interact with matter almost at all. They have no electrical charge and an extremely small, almost negligible mass. They can travel through objects like sunlight would through a clean glass window. Neutrinos can travel unimpeded from the centre of the Sun to Earth in about 8 minutes.Using incredibly sophisticated equipment, scientists have found ways to detect these neutrinos in huge underground laboratories, away from any surface radiation. This can give scientists a way to understand what’s going on in the centre of the Sun, where these particles are created.
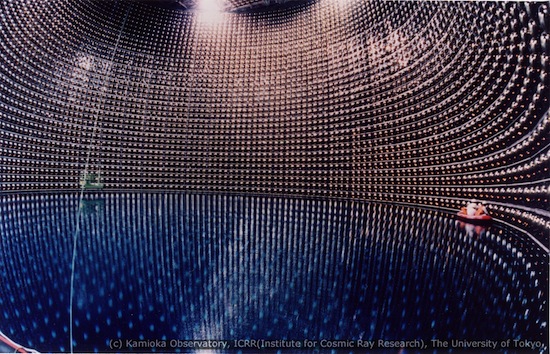
The wall of the water tank of the Super-Kamiokande Neutrino Detector in Japan is lined with thousands of photon detectors, each about the size of a beach ball. Occasionally a neutrino coming from the Sun interacts with a water molecule producing photons that are registered by one or several of these detectors. Image source: Kamioka Observatory, ICRR Tokyo
Scientists theorised the existence of the neutrino long before it was detected, and were able to even predict how many neutrinos the Sun would produce every second, and how many of those would be detectable on Earth. However, when an experiment to confirm this was carried out in the 1960s, there was a serious discrepancy. For many years scientists thought that something was wrong with our model of the Sun because only about one third to one half of the calculated number of neutrinos was registered by the detectors on Earth. This discrepancy was called the solar neutrino problem.
In order to understand the solution of this problem, one has to realize that there are three kinds (or flavours) of neutrinos: electron, muon and tau neutrinos. The nuclear reactions in the centre of the Sun only produce electron neutrinos. However, if one assumes that electron neutrinos can somehow change into muon or tau neutrinos on the way from the centre of the Sun to the Earth, this would provide an explanation for the missing neutrinos.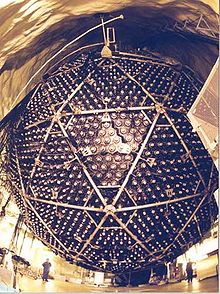
Until 2002 the terrestrial neutrino detectors could only see electron neutrinos. The muon neutrinos could not be detected by any of the previous experiments running since the 1960’s. Only in 2002 it was possible to detect electron neutrinos as well as muon neutrinos in a new detector, the Sudbury Neutrino Observatory (SNO) in Canada. It was then proven experimentally that neutrinos can change their type and furthermore the solar neutrino problem was solved because the sum of the number of detected electron neutrinos plus the number of detected muon neutrinos corresponded exactly to the prediction made by our model of the Sun.
This photo shows the large vessel that is filled with heavy water in the SNO underground laboratory in Canada. This experiment allowed to detect electron neutrinos as well as muon neutrinos and thus solved the solar neutrino problem. Image source: Sudbury Neutrino Observatory.Near the end of a Main Sequence star's life, when it runs out of hydrogen to burn, hydrogen fusion stops. Unable to produce the energy to support its own weight, the core of the star begins to collapse, increasing the pressure and temperature as it does so.
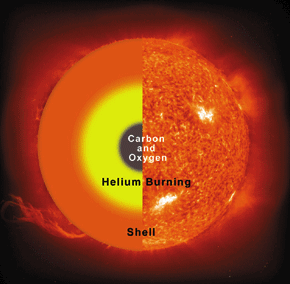
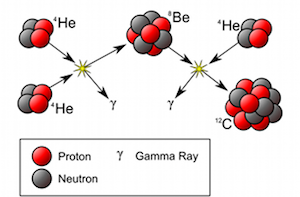
The existence and the detailed properties of this resonance were predicted by the British scientist Fred Hoyle (1915–2001) simply from the consideration that without it the carbon production in stars would not be sufficient to permit life. Only two years after his prediction, this resonance was really discovered in a laboratory experiment. The uniqueness of the triple alpha process can be seen from the fact that this seems to be the only case where the outcome of a laboratory experiment was predicted correctly on the grounds that otherwise we would not exist.
Sir Fred Hoyle is notable for a number of theories and as a writer of science fiction. He did however, feel that the universe was in a ‘steady state’ and expanded due to the creation of new matter and not the widely accepted “Big Bang” theory. Ironically, he coined the term “Big Bang” in one if his papers criticising the theory. Originally, the term was meant to mock the theory but theory supporters decided it was actually very fitting and kept it.
The prediction and then discovery of Fred Hoyle’s 12C resonance gave a lot of support to one of his other theories: the hypothesis of stellar nucleosynthesis, which claims that all the natural chemical elements are formed from hydrogen within stars.
When helium is exhausted in the centre of a star, again the core of the star contracts, rising the temperature and density so that now carbon can burn. This mechanism of contraction and ignition of a different fuel whenever the previous fuel is exhausted, repeats and leads to further consecutive burning phases, in which the products of previous burning are the fuels of subsequent burning phases. The following advanced burning phases take place, producing more and more heavy elements: carbon, oxygen, neon, and silicon. Silicon burning, producing mainly iron, is the last burning phase in a star. After that, nuclear burning cannot produce any energy anymore because the fusion of iron and heavier than iron nuclei does not release energy.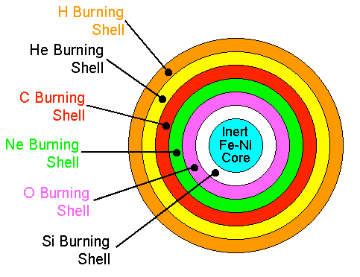
The image shows the core of a massive star at the end of Silicon burning. Typical core radius would be ~REarth while the envelope's radius would be ~5AU. Source: Richard Pogge, OSU.
Nuclear burning in the centre of stars is accompanied by shell-burning. The shell-burning processes are similar to the preceding central burning phases and occur simultaneously to the advanced central burning in spherical shells around the centre where the temperature and density are not as high but lighter nuclear fuel is still available. For example, when the core of a star is fusing carbon to produce oxygen, the shell still contains some left over helium. The energy produced by the carbon fusing core heats up the shell until it is hot enough to burn the helium. The helium fuses into carbon, which is heavy and falls into the core providing more fuel.
Not all stars can create the full list of elements enumerated above. The bigger the star, the higher the temperature the core can reach and the more likely it is to be able to make the heavier elements. Our Sun for instance, is not particularly big. At the moment it fuses hydrogen to make helium and near the end of its life it will probably manage to fuse helium and produce a carbon core; but it is unlikely to reach the temperatures necessary for fusing the carbon.For stars with less than 8 times the mass of the Sun, only hydrogen and helium burning can take place because the core of the star never reaches the temperature and density necessary to ignite another burning phase. After the helium-burning process is over, all that is left are the 2 outer shells; hydrogen at the edge, helium a little further in, and the core of the star. The core consists of the carbon and oxygen produced in the Triple-Alpha reaction. Then pulsations and strong stellar winds cause the outer layers to blow away creating a planetary nebula and leaving a White Dwarf behind.
In stars larger than 8 times the mass of Sol, the nuclear processes can continue for much longer, up to Silicon burning, leaving an iron core. When this finishes, the star cannot support its own weight and the outer shells rapidly fall into the star, then bounce back off the dense core in a powerful shockwave, leading to the characteristic explosion of a type-II supernova, that leaves behind a Neutron Star.Through these winds and explosions, the fresh elements created in the stars are distributed as gas and dust clouds into space. Thus, stars are like factories producing elements that are the building materials for new stars, planets and ultimately for us, humans.

Through planetary nebulae (left) and supernovae (right) the elements created in stars are distributed into space. Images source: NASA
The heaviest element that stars can create is iron, via nuclear fusion processes that produce the new element and release energy. However, for elements heavier than iron, the constituents are less willing to stick together. For example, if a positively charged proton tried to fuse with a positive nucleus, because they have the same charge and repel each other, instead of producing energy, such process would actually consume energy. To reach high enough energies to overcome these repulsive forces, higher and higher temperatures are thus needed.
So how are heavier elements like gold and uranium created?Well, some of these problems will be avoided when one considers neutrons. Neutrons don’t have a charge so they are able to fuse with a nucleus without having to overcome the electric repulsion. If nuclei capture neutrons, more neutron-rich nuclei can be created. If a nucleus becomes too full of neutrons some of the neutrons will transform into a proton via beta-decay. Heavier elements are produced this way.
However, they can only take place in very specific conditions. There are two processes that together are what’s known as stellar nucleosynthesis (making nuclei in the stars).The Slow or s-process: This process takes place during helium burning of Red Giants. At this phase, there is an abundance of neutrons, that get captured by other nuclei. This process is slow because there are relatively few neutrons produced and it takes million of years until an appreciable amount of heavy elements is produced. This produces elements such as zirconium, which is used as gems or in catalysts. The elements produced this way are usually stable because the process is slow enough to allow the nuclei to decay down to stability before they go on to capture another neutron.
The Rapid or r-process: This process takes place during the type-II supernovae phase. In this case the neutrons are produced by the merging of protons and electrons (electron capture on protons). It is rapid as there are a large number of neutrons produced and it takes only seconds to form a significant amount of heavy elements. This is how elements like uranium and gold are made. The new elements are formed much more quickly than nuclear decay can occur so often the elements produced this way are unstable.
Cosmic rays coming from outer space were the first high energy particles ever studied. A few cosmic rays pass through your body every second, no matter where you are. It is difficult to work out the exact origin of cosmic rays because they travel from all directions. Many originate from our Sun, others were probably emitted by supernovae. Cosmic rays striking the outer atmosphere layer are mainly fast-moving, high-energy protons. As they hurtle towards Earth, they collide with atoms in the air (mainly nitrogen and oxygen), creating new particles which shower down on to the Earth’s surface. Most of these new particles are unstable isotopes.
One of the most intriguing stories in the history of Nuclear Physics was the discovery in 1972 of a natural nuclear reactor located in Africa.
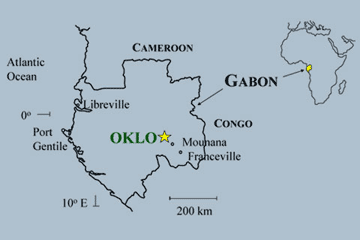
Eventually, it was found out that naturally occurring uranium had been concentrated in the rocks by geological processes to such high concentrations that nuclear chain reactions were able to start by themselves about two billion years ago and were sustained for as long as 1 million years.