The world needs a lot of energy. Technological development today requires much more energy than has been produced so far. Data shows that the larger the GNP, the larger the energy production and consumption per capita and the larger the energy production, the higher the longevity of the people. The most usual energy production is based on fossil burning (wood, coal, oil, natural gas), but these natural resources will soon be exhausted or will become prohibitively expensive. Current estimates vary from about 50 to 150 years. Sooner or later we will need other forms of energy. Could nuclear power be the answer?

We already have a great, highly efficient (more so than any other!), source of energy at hand - namely nuclear power. At present, about 16% of the electric energy produced all over the world is nuclear. This energy can cover humankind’s energy demand for thousands of years - and even better, it is estimated that breeder reactors can provide energy for up to a few billion years! The overall production cost of electric energy from nuclear power plants, including the costs of the safety systems, protection against proliferation of fissile materials, future reactor decommissioning and costs of the nuclear waste treatment and protection, has been shown to be one of the lowest among various energy sources.
Nuclar energy production does not create environmental pollution and does not contribute to global warming. It would thus be a terrible waste of resources if humankind did not make extensive use of nuclear energy. The technical problem is to use it in as safe a way as possible. For example, every 22 tonnes of uranium used for electricity saves the emission of about one million tonnes of carbon dioxide that would result through the equivalent use of coal.There are risks linked with the possibility of serious reactor failure, the ionising radiation that is released, handling of nuclear waste, and the proliferation of fissionable material. The assessment of these risks, however, is a difficult task, as they do not fall under the general class of “voluntary” risks. However, for a rough idea we can look at the “deaths per electrical energy produced”. Conventional coal mining and oil-well accidents, together with the air pollution caused by burning fossil fuels result in a risk factor that is some 40 times higher than in all of the nuclear industry, from uranium mining to potential failures of nuclear power plants, combined. A single dam failure, or simple accident at a chemical plant can kill up to thousands of people, while in what was probably the worst (and very unique) catastrophe in the whole history of nuclear power, Chernobyl, only 31 people were killed, 28 of them by large dose of absorbed radiation. The full USA nuclear program bears a risk similar to raising the highway speed limit from 80 km/hour to 81 km/hour!
During the recent Fukushima nuclear plant failure, there was a massive release of radioactive material into the environment, and experts predict that there will be a significant increase in the cases of cancer amongst the local population within the next 50 years. However, the immediate death toll due to radiation was zero.
We know by now that radioactive nuclei are produced during fission. The number of neutrons in uranium (143 or 146) by far exceeds the number of protons (92), making it relatively stable (the lifetime of 238U is 4.5 billion years, and the lifetime of 235U is 1.3 billion years). In smaller nuclei the neutron to proton ratio needed for a stable isotope is lower. Thus, when the nucleus divides into the smaller ones, the number of neutrons in every fragment is larger than what is needed for making it stable. This means that the fission fragments are unstable, meaning radioactive - and quite a lot of them have long lifetimes.
In addition to the fission fragments, transuranic elements are also produced by means of the neutron capture within the nuclear fuel. Say a fast neutron is captured by 238U. Then, after two beta decays 239Pu is formed – a fissile element that in the breeders serves as a fuel (and then we talk about uranium-plutonium cycle). However, the same isotope in a PWR-type reactor becomes nuclear waste. One must also remember that during reactor operation the construction materials of the reactor become activated (a typical product of such an activation is 60Co) and have to be handled properly.
Classification of nuclear wastes:
| Type | By Volume | By Radioactive Content |
| High Level Waste | 3% | 95% |
| Intermediate Level Waste | 7% | 4% |
| Low Level Waste | 90% | 1% |
High level wastes make just 3% of the total volume of waste resulting from nuclear generation, but they contain 95% of the radioactivity. Low level wastes represent 90% of the total volume of radioactive wastes, but contain only about 1% of the radioactivity.

Nuclear waste poses serious technological problems that must be solved in order to make nuclear power safe for the public. In contrast to coal, oil or gas, nuclear fuel never burns up completely. This is due to the fact that during the "burning" process, a number of nuclei that strongly absorb neutrons are created. With time, the neutrons produced during fission will be mostly absorbed by products of earlier reactions. Multiplication of their number in a single act of fission will not be sufficient to maintain the chain reaction. Then the fuel element can’t serve as a fuel and becomes highly radioactive nuclear waste.
Moreover, the half-lives of the elements produced in fission reaction are often as long as tens or even hundreds of thousands of years, so one has to take special care when storing such waste, and has to safely store it for a veeery long time. This is what creates serious social, political and regulatory problems for the disposal of these wastes.In research reactors that do not produce that much spent fuel, the simplest way is to use a water storage tank, usually placed next to the reactor pool. The spent fuel can be kept as long as the corrosion in the fuel cases permits, usually about 30-40 years. In the meantime the temperature of spent fuel rods decreases and natural decay processes make their activity lower. Another 40 or 50 years of storage must pass before the spent fuel activity becomes low enough for sending it to the final repository of nuclear waste.
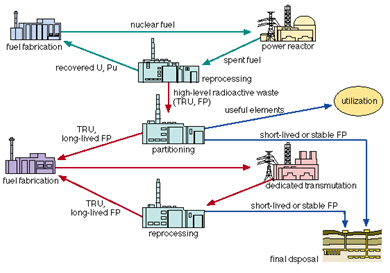
In the case of nuclear power plants similar methods can be employed. However, after a few years of keeping spent fuel in the water tank, the fuel is moved to reprocessing factories where it can undergo a chemical process in which fissile elements (uranium and plutonium and other transuranium elements) are recovered and used eventually in the production of fresh reactor fuel. The remaining material, mostly in liquid form, is vitrified, encased in huge metallic containers (casks) and sent to the repository. This technology is not very widespread, as it requires a high-tech environment. If the spent fuel is not reprocessed, it must be directly stored, in appropriate metal casks, in special repositories deep underground: for example in old salt mines, clays or granite rock.
Storage of nuclear waste at levels of 500-1000m below the ground provides higher safety than ground storage. The radiation emitted after, say, a 1000-year period, will be at the level of the natural radiation in the first 1000m of the Earth’s crust. Of course, if we will learn to transmute and incinerate nuclear waste the problem will become even easier to solve. Deep storage presents no real danger for people living near the storage sites, unless someone accidentally tries to use the terrain for some other purpose and starts drilling. Even in such a case, however, the danger would remain local, and certainly not reach global proportions.
When discussing the hazards linked to industrial nuclear waste, one often forgets that the Earth’s crust itself contains many radioactive elements that continuously diffuse towards the surface and form part of the natural radioactive background.

As the figure above illustrates, nuclear waste contributes with only a small proportion to the background radiation. For instance, all radioactive waste accumulated until the year 2000, allowed for cooling for 500 years, will show an activity equivalent to the natural radioactivity of 30x30x2km slice of soil (2 kilometres is the typical depth of an underground waste repository).
One starts with mining of the uranium ore. The ore is then crushed and milled to a fine powder. Finally it undergoes a chemical process enabling uranium to be separated from the ore. As a result, uranium oxide U3O8 is obtained. In order to run a nuclear power plant that generates, say, 1000 MW of electric power, one needs about 200 tonnes of U3O8 per year.
The next step consists in the enrichment of uranium in 235U. The process starts by converting the triuranium octoxide into gaseous uranium hexafluoride (UF6). High-speed centrifuges are used to separate the gas in two parts: the removal of 238U makes one stream enriched in 235U, while another one is depleted in 235U. The first will be used for nuclear fuel fabrication, while the latter, “depleted uranium”, can be used for example in metallic form as very efficient shield against gamma radiation.
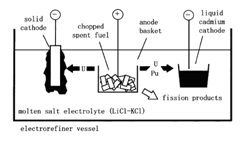
After burning the fuel in a nuclear reactor, the spent fuel is stored and then either reprocessed for recovering fissile elements (235U and 239Pu) from it or prepared for long-term storage without reprocessing.There is a strong inventive to reduce, by a factor of 100 or more, the volume and radio toxicity of High Level Wastes (HLW) designated for storage deep underground. It turns out that about 97% of the spent fuel can be recycled, the rest is left as highly radioactive nuclear wastes. The recovered uranium contains about 1% of 235U only (so-called depleted uranium). In addition to geological storage there is also interest in Partitioning and Transmutation (P&T) Technologies that would allow a separation of actinides (Pu in particular), so-called minor actinides (Np, Am and Cm) and some fission products of long lifetime, transmuting them to short-lived or even stable products.
Road transport of spent nuclear fuel in Japan (Image source: The Energy Library):
Contrary to popular belief, the transport of spent fuel is not dangerous. We note that during the last 40 years there were about 3000 transports of spent fuel recorded in the US alone. This fuel was transported by trucks and trains over a total of about 2.5 million kilometres, without a single accident occurring. Also in Europe no accidents happened during any transport of spent fuel. Safety is largely guaranteed by heavy (~120 tonnes) steel casks used during transport. Typical walls are about 50 cm thick - some 15 times more than in the case of containers used for gasoline transport. For every ton of spent fuel there is typically three times as much material used for the container and the biological shield. Such containers are built to withstand a 30-minute fire and a fall of 9 m onto concrete. They are built to even withstand a collision with a jet plane! In each container there are never more than 9-12 spent fuel elements. More recently, the construction of casks has started to be modified so as to make them resistant to possible terrorist attacks.
In addition to nuclear wastes produced by nuclear reactors and military activity, nuclear wastes are produced wherever nuclear radiation sources are used. They come from hospitals (with nuclear medicine and radiotherapy wards), from university and industrial research, from industrial use of sources (for example in the paper industry, in uranium and coalmines, smoke detectors, etc.). In contrast to the spent fuel, these wastes are entirely of low or medium activity and mostly of reasonably short lifetimes. Such wastes are usually compacted before being finally stored into special containers that prevent the leakage of radioactive material into the environment.