
The last section we did was concerned with the scale of objects,
especially the nanoscale and what we know of atoms and molecules. Now we understand the
structure of atoms, in particular the nucleus, we can study some of the more interesting
aspects of what the nucleus can do.
One of the largest branches of the study of nuclear physics is radioactivity, a natural phenomenon of certain nuclei. Radioactivity is everywhere around us; in the soil, space and even in us. We are constantly exposed to radiation, not just when we have to have an x-ray, so it is vital we understand how it works.
In this section we will discuss the discovery of radioactivity, and then go onto what causes it and where it comes from and finally how it interacts with the world around us.
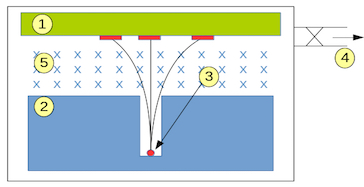
Schematic of Becquerel's setup: (1) photographic plate, (2) collimator, (3) radioactive material, (4) vacuum pump intake, (5) magnetic field. Image credits: Zoltán Elekes.
Many scientists dedicated their careers to researching this new phenomenon, including Marie and Pierre Curie, who discovered the other radioactive elements of polonium and radium. Rutherford, the scientist we have already spoken about being responsible for discovering the nuclei of atoms, also worked on radioactivity, investigating the different types of sources and their properties. It was Rutherford who coined the names for the different types: alpha, beta and gamma, after the letters of the Greek alphabet. We have already mentioned alpha particles, when they were used to probe the interior of gold atoms by Rutherford, however now we will look at them in more detail. But first, we know we can find radioactivity in sources such as uranium compounds, but where else will you find it and what is it?
We know that certain elements are radioactive, while others are not. This implies that the radioactivity is generated at the atomic
level, so we must look at atoms, in particular nuclei, to understand this better.
There are three types of radiation that result from radioactive atoms: alpha, beta and gamma radiation. These are particles or energy
emitted from the nucleus of the atom when the nucleus is unstable, or it can be said to be the decay of atoms due to instability.
So, why are certain atoms unstable and others not?
This will be explained in a later section, but for now let’s just say that it is due to the numbers of nucleons in the nucleus, in particular the ratio of protons to neutrons. The different types of radiation are emitted for different types of instability. This is why different sources emit different radiation.
Instability is not limited to nuclei. Free neutrons decay with a mean lifetime of approx 15 minutes, and even protons could decay eventually, however the half-life predicted by theory in this case is 1036 years! So on human scales, protons are considered to be stable.
Now we have an idea of what the radiation actually is, we can see where we actually find it.
Radioactivity is not limited to those dangerous elements we have discussed, like uranium or radium.
These are the strongest sources we know, but radioactivity is present in much smaller quantities in many other places. The radiation that Becquerel found coming from uranium also comes from rocks, from outer space, from air we breathe, the water we drink, the sea we swim in and our own bodies.
You may be surprised to learn that you and all the people you know are radioactive, and you may even think that this could be caused by the modern technological world we’ve developed, with nuclear power stations and computers and medical x-ray imaging, however you would be wrong. As long as there has been life on Earth, all plants and animals have been radioactive. It is part of the history of life on earth. So let’s look at where exactly we have found radioactivity in more detail.
Radioactive elements are everywhere around us in the earth. Many minerals, particularly granite, contain some uranium compounds. In fact, uranium is about as abundant in the soil and rocks as metals such as tin, zinc or tungsten. However, other radioactive compounds are much more common, such as thorium, which is about three times as abundant on Earth as uranium. In the nineteenth century, just before electric lighting took over from gas, thorium oxide was used to make gas lamps glow brightly. In the 21st century, thorium might well be a fuel in nuclear power stations.

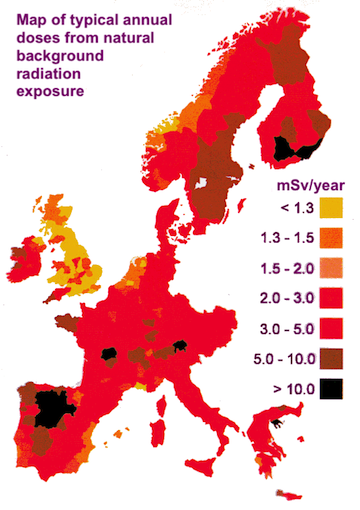
The map on below shows background radiation levels across some of Western Europe (image credits: World Nuclear Association).
The differences are mostly due to the types of rock the ground is composed of in each country. For instance, in the south west of England, the ground is made mostly of granite and so the background radiation in this area is higher than most of the rest of the country, which is made of limestone. Areas of central France have very high levels of radiation due to radon gas from the rocks. Besides the rocks, there are other factors which contribute to the background radiation we are subjected to.
In the Universe there are many sources of many types of radiation, including radio galaxies with superluminal (apparently faster than the speed of light) jets, black holes and even just the planets in our own solar system. Astronomers use specialised telescopes to look for these types of radiation, which includes x-rays and gamma rays. These sources emit in all directions and even the radiation from objects hundreds of thousands of light years away can reach us eventually. What we detect as the errant sources of radiation is called cosmic rays. You will actually measure more cosmic rays the higher you climb. On the top of mountains, far more will be detected than at sea level as they have to pass through less atmosphere.
Cosmic rays are hard to escape and are sometimes a big annoyance to scientists. Occasionally, it is necessary to measure very faint radioactivity. This could be when studying very faint, very far away gamma sources, which emit only a tiny amount in our direction or even when studying the expansion of the universe and the microwave radiation that accompanies it.

The image on the right shows a neutrino detector situated 1.4 km below the top of a mountain, in Italy.
As rivers flow over the rocks and soils, they carry with them all kinds of dissolved salts in the water. Over time, as water evaporates in the sun, the salts concentrate. Since most rocks contain some uranium, it is not surprising that the seas contain uranium salts too.
This makes the sea somewhat radioactive, and not just because of the uranium, but among other substances there is
40K (pronounced potassium-40) too.
This form of potassium is the main substance that makes our bodies radioactive.
On average, sea water contains about three milligrams of uranium in every thousand litres, i.e. every cubic metre. Not much, perhaps, but it is said that if the cost of extracting uranium from the sea could be reduced to about one tenth of what it is now, then uranium could be mined from the sea at a profit! If the present sources of uranium ever become used up, nuclear power plants might indeed run on uranium extracted from sea water.
The air we breathe contains a small amount of a radioactive form of carbon, known as 14C (pronounced 'carbon-fourteen'; you will see 14C written as carbon-14 in some books). Carbon-14 atoms are the result of the interactions of cosmic rays in the atmosphere. The cosmic rays undergo many transformations, which can include the production of thermal neutrons. These neutrons interact with the nitrogen-14 atoms in the atmosphere in a nuclear reaction that produces carbon-14 atoms and protons. These carbon atoms then go on to produce carbon dioxide molecules, which plants extract from the air in order to photosynthesise and make sugar and cellulose, thereby absorbing carbon-14 in the process. This is then passed onto us when we eat the plants, or even when we eat animals that have eaten these plants. The fact that all living things take in carbon-14 until the day they die can be used to date the remains of living things that have been in the ground for hundreds or thousands of years. This process requires thinking about the half life of the radioactive atoms, which will be covered later.
Each day the food we eat contains two or three grams of potassium. This means that, for every kilogram of body weight, something like 50 potassium atoms decay and emit radioactive particles in our bodies every second! Also remember that when we eat plants (or animals that have eaten plants) we absorb carbon-14 atoms that are also radioactive. There will also be traces of other radioactive elements, even uranium. If you happen to swallow some sea water at the beach, you will take in a tiny amount of uranium, since all sea water contains some uranium.
We've already discussed how cosmic rays produce carbon-14, which is used to produce carbohydrates when ingested by plants and therefore animals, and ourselves. Carbon-14 has then entered the food chain and all living things can be exposed to it.
Large doses of radioactivity can be deadly and thousands of people died from radiation as a result of the nuclear
bombs dropped on Japan in 1945. More recently, in 1986, 28 people were killed by radiation when
the Chernobyl nuclear power plant in the Ukraine blew up.
You can read more about these topics in the Benefits and Risks section and the History section.
The amounts of radiation that are known to damage health are far greater than the background radiation from cosmic rays, rocks etc. Any of our cells that might be damaged by these sources can repair themselves, as only a few will be damaged. For larger doses of radiation, a large number of cells are damaged beyond repair, which is why it can be dangerous.
We know that plants and animals have been living and evolving with radioactivity for billions of years. So whether radioactivity is dangerous depends on how much we receive. Even too much common salt can kill people, which is why people adrift on the ocean can die of thirst! As they say, too much of anything is a bad thing.
So how do we know how much radiation is ‘too much’? Firstly we need to understand the various types of radiation and how dangerous each is. We will discover the level radiation must be at to be dangerous in our section on activity and interaction with matter.
We found out what radioactivity actually is in the previous section: it is the decay of unstable atoms and the subsequent emission of a particle or energy. Now we will look at what makes an atom unstable and what types of radiation there are.
We know that atoms consist of a nucleus made of protons and neutrons with electrons orbiting the nucleus. We also know that atoms are electrically neutral, while ions, which have lost or gained electrons, are charged. The key to understanding radioactivity is in isotopes.
We have talked about carbon-14 being radioactive, while the regular carbon-12 is not. So how is carbon-14 different to carbon-12 ?
The difference lies in their nuclei. For both atoms to be fundamentally carbon they must have the same number of protons, or the same
atomic number. It is this that makes an element what it is. If we change the number of electrons the atom has it only makes the atom into an ion.
Therefore we must change the number of neutrons. So we say that carbon-14 is an isotope of carbon-12, and merely has a difference of
having an extra two neutrons in the nucleus, so a different mass but the same element overall.
There are many isotopes of every element. A famous example is deuterium, an isotope of hydrogen with one neutron and one proton. This can be used to make heavy water, which has many interesting uses including detecting neutrinos and moderating nuclear reactors.
Unstable isotopes are often very useful in medical procedures, and can be safely used because of their short lifetimes before they decay. A problem with this is that these substances cannot be found naturally on Earth and therefore must be manufactured in the hospitals in machines called cyclotrons, which accelerate atoms to extremely large speeds along a circular path using electromagnets. Cyclotrons are available in many different sizes, depending on the usage and the isotope being produced. In the machines nuclei are collided with charged particles and the necessary radioisotope is produced. The term radioisotope is used for isotopes that are unstable and radioactive.
The most stable versions of elements are the ones we list in the periodic table, as in the ones we find naturally and most commonly. However, they are only really common because of their stability; the unstable isotopes have decayed into other more stable elements, leaving the stable versions. So what makes one isotope more stable than another?
In the section concerning nuclei, be briefly mentioned that the nucleons were held together by the strong force, which overcame the electrostatic repulsion between the protons. This is the key to understanding the stability of nuclei. Both neutrons and protons are affected by the strong force, however only the protons act to repel each other, therefore the neutrons act to strengthen the force holding the nucleus together without adding to the electrostatic repulsion.
You may think that the more neutrons a nucleus has, the more stable it should be after what we have just discussed. However, this is not the case. Nuclei are only stable for a specific range of ratios of neutrons to protons, about 1-1.6.
Outside these ratios, the nucleus will be unstable and decay. For example, nitrogen is stable and has a ratio of 1: it has 7 neutrons and 7 protons. On the other side of the range, lead, which is also stable, has 82 protons and 126 neutrons, with a ratio of 1.54.
A graph showing the region of stability according to numbers of protons and neutrons is shown below, where Z is the number of protons in a nucleus,
N is the number of neutrons, A=Z+N is the number of nucleons, and the two curves correspond to (1) stable nuclei, (2) P=N line.
Graph credits: Zoltán Elekes.

A very important aspect of nuclear physics is binding energy. There are several types of binding energy but for our purposes we will look at nuclear binding energy. This is the amount of energy you would need to put into a nucleus for the nucleons to overcome their attraction and be separated into individual nucleons. The binding energy of a whole nucleus is lower than for the sum of its constituents, which is another way the nucleus is held together. It is more energetically favourable (i.e. the energy is lower) for nucleons to form and stay in nuclei rather than be separate. One concept within this branch is binding energy per nucleon.
There is a limit to the mass of a nucleus at which the strong force will no longer be able to hold on to the outermost nucleons. The largest nucleus known is that of uranium, which has 238 nucleons. However, even this nucleus is highly unstable, as we know uranium is radioactive, therefore it must undergo decay.
Alpha decay allows the nucleus to lose mass to improve stability by emission of an alpha particle, or helium nucleus. The emitting nucleus will lose four nucleons, two protons and two neutrons, and undergo transmutation due to loss of protons. These are the alpha particles that were used in Rutherford’s scattering experiment to probe atoms.
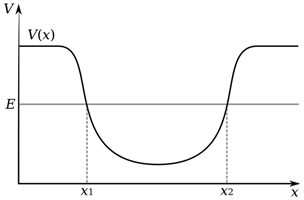
As we have said, alpha decay acts to decrease mass, however it is only feasible when binding energy per nucleon is not at a minimum, so for elements with nuclei heavier than nickel, however, it has only actually been observed for the most massive nuclei of element tellurium and above. Alpha decay is an example of a process called ‘quantum tunnelling’. The nucleus of an atom creates a well of potential energy, as shown in the diagram, which traps the alpha particle that already exists inside the nucleus.
One of the first characteristics used to differentiate between the types of radiation was how much material it could penetrate. Alpha particles are massive and have relatively low speeds, so are very likely to interact with any particles they come into contact with. This means they can only penetrate through a few centimetres of air, or a thin sheet of paper or aluminium.
There are many alpha sources, one of the most famous being uranium. Uranium decays by emitting an alpha particle (α) and transmutes into thorium, as shown by the equation below:
238U → 234Th + α
This is only the first step in the decay of uranium, as it will have to progress through many other stages to become completely stable.