
Comparisons between the observed abundance of certain naturally occurring radioactive isotopes and their decay products, using known decay rates, can be used to measure timescales ranging from before the birth of the Earth to the present. For example measuring the ratio of stable and radioactive isotopes in meteorites can give us information on their history and provenance.

Radiometric dating techiques were pioneered by Bertram Boltwood in 1907, when he was the first to establish the age of rocks by measuring the decay products of the uranium to lead.
Today, radiometric dating is considered a very reliable dating method, and the principal source of information about the absolute age of rocks and other geological features, including the age of the Earth itself. The techniques can be extended to date a wide range of man-made materials as well. Because radioactive elements have various half-lives, there are numerous different methods that apply to different timescales. Among the best-known techniques are radiocarbon dating, uranium-lead dating and potassium-argon dating.Carbon is the basic building block of organic compounds and is therefore an essential part of life on earth. Natural carbon contains two stable isotopes 12C (98.9%) and 13C (1.1%), plus a tiny amount of the radioisotope 14C (1.2 x 10-12%) with a half life of 5730 years. 14C is produced by cosmic rays in the atmosphere and then distributed in nature (93% in the ocean, 5% in the biosphere and 2% in the atmosphere).
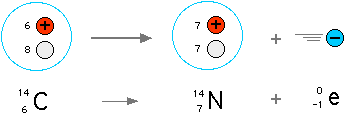
Radiocarbon dating was developed in the 1950s, with Willard Libby receiving the 1960 Nobel Prize in chemistry for the use of 14C to determine age in archaeology, geology, geophysics and many other branches of science.

A mass spectrometer coupled to an accelerator is used to date samples containing only a few miligrams of carbon by measuring the ratio 14C/12C (Image courtesy of J. Forest, CNRS Photothèque).
However, radiocarbon dating does have its limitations. Carbon is only absorbed by living organisms (or rather things that were alive at some time) such as wood or fossils so it can’t be used to date stone or pottery for example, and the method is accurate only for objects up to about 60,000 years old.For many years it was assumed that the content of 14C in the atmosphere was constant. We now know that the Earth and solar magnetic fields are changing in time. This means that the flux of cosmic rays impinging on the atmosphere varies, and therefore so does the 14C production rate. That makes it necessary to calibrate the 14C dates according to other techniques. One such technique is the dendrochronology, or tree-ring dating.
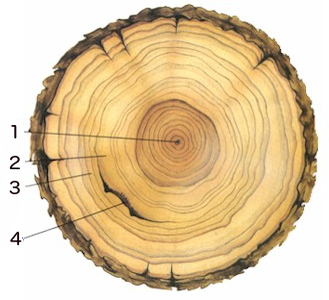
The dendrochronology involves obtaining a horizontal cross-section of the main trunk of a tree and analysing the visible rings caused by the natural plant growth. These rings result from the change in growth speed through the seasons of the year, with each ring usually marking the passage of one year in the life of the tree. This technique works best in temperate climates where the seasons differ more markedly, and, obviously, one can only date back a few hundred years as very old trees are rare.
Many prehistoric cavern paintings dating up to 30,000 years ago, have been investigated using radiocarbon dating, because the materials used are mostly of organic origin.

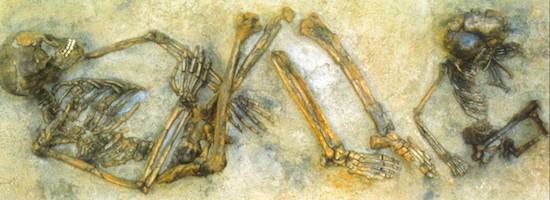
Radiocarbon dating can even be used to date more unusual archaeological finds. In September 1991 two mountain hikers discovered the body of a man sticking half-way out from the ice in a mountainous region of the Alps. The iceman, later nicknamed Ötzi after the mountain range where he was found, might have died more than 4000 years ago.

Detailed 14C measurements were performed independently by four different institutes on bones and tissues from Ötzi (in Innsbruck, Bolzano, Zurich and Oxford), on equipment and materials apparently belonging to the iceman (at Uppsala, Gif/Yvette and Vienna) and on sediments collected from the discovery site (at Vienna). The uncalibrated age is 4550 years. When translated into the calibrated age with the help of tree-rings technique curves, Ötzi appears to be about 650 years older. So Ötzi died between 3350 and 3100 BC.
Analysis of the corpse revealed astonishing detail about his life. The analysis of cereals found in the bowels as well as of minerals in the hair led to the conclusion that Ötzi was coming from south Tyrol when trapped by snow and ice. He had eaten porridge of einkorn (a type of wheat), vegetables and meat recently before his death. Moreover, by analysing the isotopes of carbon and oxygen in the teeth and bones of the iceman, the researchers were able to differentiate the country of his early childhood from that where he lived later. We also know that he was a 45 year old man with shoulder length, dark, wavy hair and he had blue eyes. However, more recent genome analysis suggests that Ötzi was original from Sardinia!
The tree-ring technique is useful for calibrating the 14C method up to about 11,000 years. For ages between 10,000 and 30,000 years, the calibration technique used is Uranium-Thorium of lake sediments and coral.

During their lifetime, corals absorb the uranium (with a half life of 245.5 years) present in the seawater but they do not absorb the thorium (230Th). After the corals die, the Uranium decays into 230Th, which accumulates in their skeleton. Measuring the ratio U/Th can give an indication of the coral's age.
However, thorium is also radioactive and decays (half life 75,380 years) into other elements via a long decay chain, finally ending with lead, which makes the dating process a little more complicated. The U-Th method can be used to date subjects with ages ranging between 10,000 and 500,000 years.However, the method has some limitations. Recent studies have shown that lead can be produced via neutron capture and may not have originated from uranium decay. This would change the dating results.
Coral used as reference for the U-Th dating method. Image courtesy of J. Lecomte (CNRS Photothèque).
Thermoluminescence was discovered by Sir Boyle in 1663. Many crystalline materials such as some minerals have this property of emitting light when heated. Three hundred years after this discovery, scientists have learned how to explain this effect. When the material is exposed to high energy radiation, the electrons in the material move into an excited state. In some minerals, this energy is then trapped inside due to defects in the crystal lattice. But when the crystal is heated, the electrons can drop into the lower energy shells, emitting a photon at each such transition.

It was shown that the intensity of the emitted light is directly proportional to the radiation that the mineral has received. Thus, thermoluminescence can be used for dating objects that have been exposed to cosmic rays or to radiation from the ground, since the doses depend on the object's age.

This technique has many applications - such as the dating of heated flint, pottery and ceramics of prehistoric periods. The oldest artefacts that can be dated by thermoluminescence are around 250,000 years old.
Above: radium radiation gives a blue tint to a glass recipient from Pierre and Marie Curie‘s laboratory. Image courtesy of C. Delhaye (CNRS Photothèque).Left: pottery dated by thermoluminescence to be 7,000 to 10,000 years old. Image courtesy of A. Chênè (CNRS Photothèque). Below: Skeletons of a woman and of a child dated by thermoluminescence to be 92,000 years old. Image courtesy of CEA – CNRS – CFR.

The K-Ar method is well suited to study volcanic activity, and given that 40K has a half-life of 1.3 billion years, the method is used in geochronology to date periods ranging from 106 to 109 years back. Radioactive 40K is common in micas, feldspars, and hornblendes, which have fairly low closure temperatures (from 125°C for mica to 450°C for hornblende).
The K-Ar method was used to determine the succession of geological periods on Earth, to fix the age of Earth at about 4.5 billion years and to pinpoint the beginnings of mankind (in east Africa) at around 5 million years ago. The method is also employed to measure the reversal rate of the Earth's magnetic poles.
87Rb decays to 87Sr with a half life of around 48 billion years. The other Sr isotopes (A = 84, 86, 88) are stable, so strontium is a useful tracer for the age and rubidium content in rocks, and has also been used to date lunar samples. The ratio 87Sr / 86Sr varies from 0.703 in young rocks to 0.750 in the oldest ones. With the 87Rb - 87Sr method it is possible to date materials aged between 10 million and 10 billion years.
An interesting story is how Rb-Sr dating helped to determine the origin of the king Yax K’ uk Mo, who founded the dynasty that ruled the Mayan city of Copán (presently in Honduras) for 400 years.
In the 8th century, a modest village in Mesoamerica by the name of Copán (located in present-day Honduras) became one of the most important Mayan cities. This rise in importance is strongly attributed to Yax K’ uk Mo, who arrived in Copán in the year 427.
At the time, Teotihuacan was at its height, the largest city in pre-Colombian Mesoamerica of the valley of Mexico. Cities hundreds of miles away copied its style of temples and adopted the same gods. Artisans from Copán depicted Yax K’ uk Mo with Teotihuacan trappings, apparently indicating the king's origins and association with Teotihuacan. The origins of Yax K’ uk Mo remained vague until measurements of the isotope ratios 87Sr / 86Sr and 18O / 16O in the teeth and bones of his remains in Copán were performed.
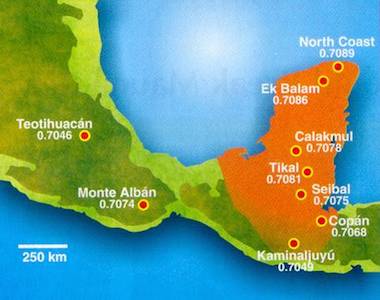
The Yucatan peninsula where king Yax K’ uk Mo lived and died is made mostly from marine sediments. It shows a smooth variation of the ratio 87Sr / 86Sr (between 0.7049 and 0.7089). The valley of Mexico is made of volcanic rocks, and there the variation is larger, while at Teotihuacan, the value is lower (0.7046). Tooth enamel provides a durable coating for the dentin underneath. Both contain calcium but they were formed very differently. Enamel mineralises when the tooth grows in the childhood, dentin as bones form continuously during the life. Strontium is chemically close to calcium and can replace it in enamel and dentin of the teeth and in the bones. Strontium is absorbed from food, and the ratio 87Sr / 86Sr will reflect the nature of the local soil. Thus a person's tooth enamel bears the isotopic signature of the food and water of their early childhood country, isotope ratios in dentin and bones reflect their later whereabouts. At 0.7084, the ratio 87Sr / 86Sr in enamel taken from a tooth of Yax K’ uk Mo was corresponding to the value at Tikal, north of Copán.
Right: ceramic figurine of the king Yax K’ uk Mo depicted with Teotihuacano trappings (Copyright American Institute of Physics 2004).
Left: measurements of the ratio 87Sr / 86Sr for various pre-Colombian cities in Mexico and Yucatan peninsula (Copyright American Institute of Physics 2004).
The isotopic ratio 18O / 16O can provide another location indicator. Oxygen enters the body through the water supply, and rain from clouds near the ocean is richer in 18O than the rain from clouds that have travelled far inland.The isotopic analysis of the strontium and oxygen ratios in the king Yax K’ uk Mo’s tooth enamel and bones did not support a Teotihuacan origin. Instead, it was concluded than the he spent his early years near Tikal and moved later to Copán.
