You may have already heard of something we call an element. Every material we have in our everyday lives, including our own tissue and bones, is made of these elements, and there is a surprisingly small number of them.
What makes one element different from another?
This question will be answered more fully when we go on to explain about the structure of atoms in further detail.
Elements differ at the atomic scale; gold has different properties than magnesium because individual atoms of
the material have slightly different structures. This difference can affect physical properties, such as the
appearance of the element in its pure form (for example gold is a shiny, hard, yellow substance, while magnesium
is silvery and more flexible), and most importantly, chemical properties, or how they interact with other
substances (gold is extremely unreactive, while magnesium will react readily with water).
Their structural differences also affect how they combine with other elements.
There is a useful tool, called the Periodic Table, which organises the elements according to their
atomic structure, and consequently, their properties. This will also be looked at in more detail later.
The elements that make up everything we see cannot be produced synthetically, but are rather found naturally. So where did all the carbon we are made from come from? All of the stable elements that we see actually originated far beyond the Earth, in space. The light elements up to iron, are produced in a process called nucleosynthesis in the cores of stars. Elements above this are made when the stars die in giant explosions called supernovae. Both of these processes will be explained in much more detail in a later section. This may give you a clue as to why there is more carbon that gold in the Universe, if you consider that only the most massive stars can burn heavier elements such as gold and undergo supernovae.
Atoms have been theorised to exist since the days of the ancient Greeks, when the philosopher Democritus
realised that at a certain point, he could not cut his bread any smaller: it was indivisible. He suggested that
everything must be made up of some fundamental blocks, which cannot be destroyed, and he named them after
the Greek word ‘atomos’, meaning ‘no cut’.
However, a more modern idea of atoms began with the advance of chemistry. John Dalton noticed that 8 grams of oxygen always combined w
ith 1 gram of hydrogen exactly. This led to the theory that a certain number of objects on a tiny scale of one
element must combine with a certain number of another, and each of these objects was different for each element.
This evolved into the idea of atoms. While the chemists had the basic idea of atoms, it was the physicists who discovered
that there was more to the atom than they first thought.
The discovery of the nucleus
When atoms had become an accepted idea of the structure of materials at a very small scale, scientists began to
theorise about what gave an atom its properties; why gold atoms behaved differently than magnesium atoms. One of the
most widely accepted theories was that of the ‘Plum Pudding model’. In this model, the atom was a sea of
positive charges interspersed with negative charges, which then cancelled each other out so that the atom was neutral.
The first evidence that the real structure was completely different was found in 1911 in an experiment conducted by Hans Geiger
and Ernest Marsden at the suggestion of Ernest Rutherford.
They famously bombarded a sheet of gold foil (only a few atoms thick) with what they knew as alpha particles: positively
charged particles which are today known to be helium nuclei. They found that some of the particles rebounded
straight back at the detector after hitting the foil, instead of passeing straight through or being deflected at slight angles
as expected based on the ‘Plum Pudding model’.
This inspired Rutherford to conclude that the atoms actually have a very small volume
of positive charge in the centre, which caused the alpha particles that hit it head on to rebound. He also realised
that much of the atom must be comprised of empty space for the majority of the particles to pass straight through.
Those particles that were deflected slightly must have been passing very near to the positive charge at the centre and
were repelled by like charge.
His final model consisted of a tiny ball of positive charge in the centre of the atom, which he deemed the nucleus,
and to ensure that the atom was neutral, negative electrons orbiting this in a similar way to planetary orbits.
This model was the accepted theory until slightly more modern quantum physics experiments showed that this was not
entirely correct.
The nucleus
As the title suggests, nuclear physicists concentrate on the behaviour of the nuclei of atoms, so it is key for them to
understand its structure. Electrons had already been discovered by J.J. Thomson when he showed that cathode rays actually
consisted of negatively charged particles, whose exact charge/mass ratio was measured later in Millikan’s experiment
on charged oil drops. It had been suggested many years before Rutherford’s experiment that all atoms were made of
hydrogen atoms, which was almost correct. In 1917, Rutherford carried out an experiment that involved firing
alpha particles into pure nitrogen gas. His detectors showed the
signals for hydrogen nuclei and he concluded that they must have been emitted from the nitrogen nuclei after collisions
with the alpha particles. He decided that this meant that the nitrogen nucleus contained hydrogen nuclei, or protons.
This is considered to be the first recorded nuclear reaction.
At this point, scientists had developed the concept of mass and atomic numbers.
The mass number is the actual mass of the atom (we can actually assume it is the mass of the nucleus, as electrons have negligible mass)
and the atomic number is the number of protons, or the number of electrons in the atom.
The existence of the neutron was first suggested by Rutherford in 1920 to account for the discrepancy between the atomic
numbers and mass numbers of nuclei. However, it was James Chadwick in 1932 that showed that radiation previously thought
to be gamma rays was actually comprised of neutral particles of mass approximately equal to that of the protons.
The particles were named neutrons and fitted into the nuclear theory as the missing mass.
The collective term for protons and neutrons, both of which reside in the nucleus is nucleons. The nucleons are held
together by the strong force, which is one of the four fundamental forces in the universe. This particular force is
approximately 1038 times stronger than gravity,
only acts within a distance of 10-15 m, and overcomes the electrostatic repulsion between the like positive charges of the protons.
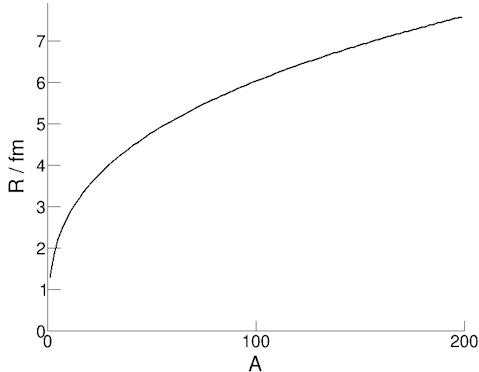
We can find the radius of a nucleus by scattering electrons off the nuclei and studying the pattern. While we can make a
calculation using conservation of momentum in this scenario, experiments have shown that the equation for the radius canbe approximated with the formula:
R = r0 A1/3
where R is the nuclear radius, A is the number of nucleons and r0 is a constant (the radius of a nucleon ≈ 1.3 fm).